Equilibrium Physics at Extreme Conditions
Mission Statement
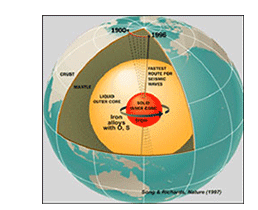
A physics-based understanding of how materials behave under high pressure, high temperature, and high strain rate conditions is at the heart of all Department of Energy (DOE)/National Nuclear Security Administration (NNSA) national laboratory programs. Consequently, Lawrence Livermore National Laboratory (LLNL) is a world-leading institute for high pressure materials research with an integrated program of static high pressure shock wave research, high power lasers, condensed matter theories, and computational capabilities. The High Pressure Physics Group leads the experimental static high pressure program at the Laboratory in support of DOE, Department of Defense (DOD), and LLNL programs, including NNSA Campaign-2, the Physics Data Research Program, the Laboratory Directed Research and Development Program, DOE's Basic Energy Science organization, and DOD's Office of Munitions.
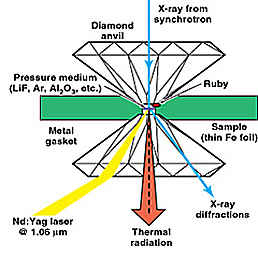
The research of the High Pressure Physics Group focuses on scientific challenges in condensed matter under extreme conditions of pressure and temperature, including the synthesis and characterization of novel materials. The group uses state-of-the-art experimental facilities and technologies such as third-generation synchrotron sources and laser spectroscopic technologies. Materials of particular interest to the group include f-electron rare earths and actinides, d-band transition metals and transition metal compounds, low-Z elemental solids, and novel superhard and high-energy-density materials of scientific and programmatic importance.
The group consists of highly talented and motivated scientists at all levels: researchers, post-doctoral fellows, pre-doctoral students, and technical and administrative personnel. Much of the research conducted is carried out in a collaborative manner among those scientists and their colleagues.