The fundamentally derived determination, prediction, validation, and accurate tabulation of the high pressure response of materials are critical in the assessment of stockpile performance. High pressure properties of materials can be broadly classified in terms of:
- Thermodynamic properties (equation of state [EOS], melt, structures, phase diagram of d- and f-transition metals—Fe, actinides, simulants, alloys, and low-Z stockpile materials like H2, DT, Be, foams): data for multi-phase EOS models for Pu and also for Earth and giant planetary models
- Mechanical properties (Strength, elastic properties, characterization of actinides, simulants, and alloys): data for the multi-scale strength model and for materials science
- Electronic and phonon properties (phonon density of states and dispersion, Heat capacity, Gruneisen gamma of actinides and lanthanides): fundamental physical data of highly correlated d- and f-electron systems
- Chemical properties (EOS, stability, structure, kinetics of high explosives and major detonation products): Data for high explosives EOS/kinetic models and also for new chemistry and planetary models
Thermodynamic Properties
High pressure thermodynamic properties such as the equation of state (EOS), melt, and phase diagram of solids establish fundamental relationships between thermodynamic variables (pressure, density, temperature, etc.) and provide closure of the hydrodynamic equations which explicitly ensure conservation of mass, energy, and momentum. Hydrodynamic simulations require thermodynamic input data in every zone and for every time step. Importantly, the predicted performance can be very sensitive to materials EOS and phase boundaries. In addition, materials models are used to determine the dynamic mechanical response in regimes of high pressures and energies. These materials models apply equally well to performance as well as reliability and safety issues. Consequently, comprehensive and in-depth knowledge of the high pressure thermodynamic properties of materials is absolutely essential to all simulations of stockpile devices.
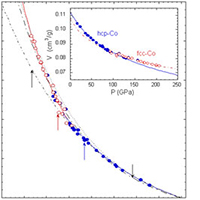
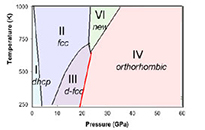
For example, we have studied the phase diagram of 3d-magnetic transition metals (Fe, Co, Ni) to multi-Mbars and several thousand degrees by in-situ diamond anvil cell x-ray/laser-heating experiments using intense synchrotron x-rays (see Yoo, et al., Phys. Rev. Lett. 84, 4132 (2000) and Science 270, 1473 (1995)). In this study, we discovered a new non-magnetic phase of β-Co, provided a critical constraint for the γ/α/liquid-triple point of iron and found a systematic change of crystal structures in these magnetic 3d-transition metals at high pressures. These results are important for understanding the Earth-core, yet the experimental technologies developed in the study have enabled the studies of stockpile materials at the pressure-temperature conditions of programmatic importance.
Mechanical Properties
Developing a predictive constitutive model for stockpile materials requires knowledge of mechanical and metallurgical properties at various length scales from atomistic to meso- and macro-scales. A major uncertainty in current constitutive models is the lack of knowledge concerning material strength and deformation near melting. Transition metals and alloys exhibit notoriously complicated metallurgical behavior, particularly when these materials undergo melting and phase transitions at high pressures and temperatures. These effects include phase separation, quenching of metastable phases, super-heating (or cooling) of solid (or liquid), elastic and plastic deformation, all of which alter the mechanical properties of materials in significant ways. In this study, we investigate the elastic constants, strengths, and microstructures of d- and f-band transition metals that have been exposed to various cycles of pressures and temperatures across the melting and phase boundaries by laser-heating at high pressures. Various x-ray technologies will be employed for the study, including the stress- and angle-resolved x-ray diffraction (SAX), x-ray flow stress measurements, and x-ray tomography, all using high quality, intense micron-size x-ray beams from third generation synchrotron sources.
Martensitic phase transitions are common in f- and d-band metals as well as rare-gas solids at high pressures and are important for understanding the mechanisms and dynamics of the transitions. Recently we have determined the incipient growth of hcp-Xe from fcc-Xe by using angle-resolved x-ray scattering at the SSRL and ESRF (see Cynn et al., Phys. Rev. Lett. 86, 4552 (2001)). The diffuse scattering in the fcc-Xe lattice is an indication of lattice distortion, which may result in an unusual melting curve of Xe, different from that of corresponding states.
Electronic Properties
Unusual phase transitions driven by electron correlation effects occur in many f–band transition metals and are often accompanied by large volume changes: ~ 20% at the δ–α transition in Pu and 5–15% for analogous transitions in Ce, Pr, and Gd. The exact nature of these transitions has not been well understood including the short-range correlation effects themselves, their relation to long-range crystalline order, the possible existence of remnants of the transitions in the liquid, the role of magnetic moments and order, the critical behavior, and the dynamics of the transitions among other issues. Many of these questions represent forefront physics challenges central to stockpile materials and are also important in understanding the high pressure behavior of other f- and d-band transition metal compounds as well as insulator–metal and molecular–nonmolecular transitions in simple molecular solids.
Recently, we have studied the electronic phase transition from Pr-III(dist.-fcc) to Pr-IV(orthorhombic) at 20 GPa. This transition is accompanied by a large volume collapse, 10.2 % at ambient temperature, as a result of delocalization of 5f-electrons at high pressures. The temperature dependence of the transition, indeed, suggests that the volume collapse may be due to disappearance of magnetic moment in the high density phase (see Baer et al., Phys. Rev. B 67, 134115 (2003)).
Chemical Properties
Application of very high pressure significantly alters the nature of intermolecular interaction, chemical bonding, molecular configuration, and crystal structure of solid. Experimental and theoretical determinations of the material response at high pressures and temperatures are thus critical for understanding thermal, mechanical, chemical, and electronic properties of solids. Current material theories or models describe some properties very well; others are at best qualitatively understood. Too frequently, relevant properties have not been measured; and models must be compared with large extrapolations of trends from very different pressure, temperature, and time scales. For example, simple molecular solids like C, CO2, N2, O2, H2O, CO, NH3, CH4, H2 are typically very stable at ambient conditions. These molecular forms are, thus, often considered to remain in stable forms at extremely high pressures and temperatures. The high stabilities of these covalent molecules are also a basis on which their mixtures are often presumed to be the major detonation products of energetic materials as well as the major constituents of giant planets. However, their stabilities are not truly understood at the extreme of pressure and temperature. Indeed, an increasing amount of experimental evidences contradicts the assumed stability of the molecular forms of these materials at high P–T conditions. For example, molecular carbon dioxide polymerizes above 40 GPa and 1800 K; liquid carbon may undergo a first-order phase transition; shock-compressed hydrogen metallizes at around 140 GPa. The objective of this program is, therefore, to investigate the stability and structure of simple molecular solids in situ at the high pressure–temperature conditions of energetic detonation and/or giant planetary interiors by using state-of-the-art spectroscopic and x-ray technologies applied to CW and pulsed-laser-heated samples in diamond anvil cells.