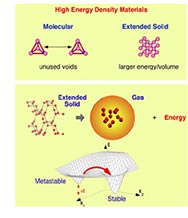
High Energy Density Materials
The High Pressure Physics Group is engaged in an experimental effort to achieve the synthesis and characterization of members of a new class of energetic materials that are predicted to have energy densities (energy per unit volume) much larger than conventional high performance explosives. Conventional energetic materials are characterized by strong intramolecular bonds (within molecules) and weak intermolecular bonds (between molecules), leading to large equilibrium volumes for the liquid and solid phases. In contrast, the class of novel energetic materials we are investigating achieve an unprecedented enhancement in energy density by replacing weak intermolecular interactions with highly energetic covalent bonds. These novel materials, known as extended solids, represent the analog of infinitely large, energetic molecules.
Calculations performed at Lawrence Livermore National Laboratory (LLNL) predict the existence of extended solids with dramatically enhanced energy densities (E/V) of about 3 times that of HMX (see C. Mailhiot, L. H. Yang, and A. K. McMahan, Phys. Rev. B 46, 14419 (1992)). These novel materials constitute a new class of materials, largely unexplored, that are thermodynamically stable at high pressures. Thus, the extended solid phase can be synthesized using various high-pressure methodologies we have developed at LLNL. These new phases represent normally gaseous systems that have been transformed into fully interconnected covalently bonded, three-dimensional solids. Several of these extended solids are predicted to be metastable and recoverable at ambient conditions [see T. W. Barbee III, Phys. Rev. B 48 9327 (1993)].
Conventional energetic systems are condensed into liquids or solids by weak, non-energetic van der Waals interactions, while the extended solid systems replace weak intermolecular attractions with energetic covalent bonds. Thus, though the energy per unit mass is approximately the same as in conventional energetic materials, the novel extended solids contain dramatically more energy per volume. The goal of this project is to identify and to synthesize such novel materials, characterize their properties, and investigate technologically viable pathways for bulk characterization and synthesis.
Our research is focused on common atmospheric gases including carbon monoxide, carbon dioxide, and nitrogen. In the case of carbon monoxide, we have used a wide range of optical diagnostic probes to study the nature of the high pressure and recovered products [M. J. Lipp, et al., J. Low Temp. Phys., 111, 247 (1998)]. Studies of carbon dioxide have been very successful, leading to the discovery of new phases [C. S. Yoo, et al., Phys. Rev. Lett. 83, 5527–5530 (1999)]. We are attempting to recover the high-pressure extended-solid phase of carbon dioxide to ambient conditions. Through the addition of dopants or catalysts, the metastability may be extended to ambient conditions. Results to date are promising and suggest that theoretical predictions will be borne out by the experimental efforts.
Super-Hard Materials
Application of very high pressure strongly perturbs chemical bonds, electronic and crystal structures, thermal and mechanical properties, and reactivities of solids. These perturbations provide opportunities for synthesizing novel materials. Typical materials of interest are C, CO2, N2, O2, H2O, CO, NH3, CH4, H2, and other molecular compounds of first- and second- row elements. Many of these materials are characterized by strong intramolecular covalent bonds which typically make molecular species very stable at ambient conditions, but not at high pressures and temperatures. We have recently shown that c-BN can be formed directly by reacting elemental boron and nitrogen. Such direct elementary reactions typically proceed very exothermically, which even accelerate the reaction. Various forms of novel oxides, nitrides, hydrides can be formed in similar elementary reactions, including diamond, c-BN, b-Si3N4, B2O3, various polymeric forms of C/N/O compounds.
A direct elementary reaction between boron and oxygen at 6 GPa yields an optically transparent B2O3. Similar reactions of B with N2 also yields c-BN and h-BN, depending on the P,T conditions.